Staying up-to-date on the optimized anchor drug of RA therapy methotrexate
This year´s annual meeting of the European Alliance of Associations for Rheumatology (EULAR) took place in Milano from May 31st to June 03rd. The symposium "Improving first-line therapy of RA patients with optimized methotrexate” was very well attended. In addition to an introductory lecture on the therapy of rheumatoid arthritis (RA) with subcutaneous (SC) methotrexate (MTX), 3 further lectures with new publications on the active substance MTX - including the SC administration - were presented here.
Worldwide, methotrexate (MTX) has been established as primary disease-modifying drug in numerous rheumatic diseases, e.g., for rheumatoid arthritis (RA). Current international therapy guidelines for RA still recommend methotrexate (MTX) as the first-line treatment. This is mainly based on its reliability with respect to efficacy, safety (especially in the presence of folic acid) and possibility to individualise doses.1
As in previous years medac organised a satellite symposium at the annual congress of the European Alliance of Associations for Rheumatology (EULAR). The introductory lecture has been given by the moderator of the symposium, Prof. Torsten Witte from Hanover, Germany followed by talks by Prof. Yoshiya Tanaka, Kitakyushu, Japan, Prof. Andrea Rubbert-Roth, St. Gallen, Switzerland and Dr. Alessandro Giollo, Padua, Italy.
Clinical benefit of methotrexate and the subcutaneous route for RA therapy
In the beginning of his presentation Prof. Torsten Witte covered the mode of action of MTX, its efficacy and safety. Furthermore, he deepened the beneficial effects (better bioavailability, better efficacy, reduced gastrointestinal adverse events) 2,3,4 of SC administration of MTX versus oral. He showed, based on the Braun study 5, that SC administration was significantly more effective than oral administration at the same MTX dosage. In addition to the advantages mentioned, a retrospective cohort study 6 published by Harris showed that use of SC MTX is associated with longer duration of MTX monotherapy before addition of other DMARDs/biologic agents in RA patients.
Particularly noteworthy was the insight Prof. Witte gave into a German health insurance data titled "Methotrexate supply before the use of biological drugs in rheumatoid arthritis" 7 that showed that the RA therapy does not appear to be in accordance with the guidelines: 65% of the patients received an MTX dose of < 20 mg/week. The maximum dosage of 20-25 mg/week was only reached in about 35% of patients in Germany. Conclusion of Prof. Witte: “In daily practice in Germany, the MTX dose is not optimised in every RA patient. There is still room for improvement.”
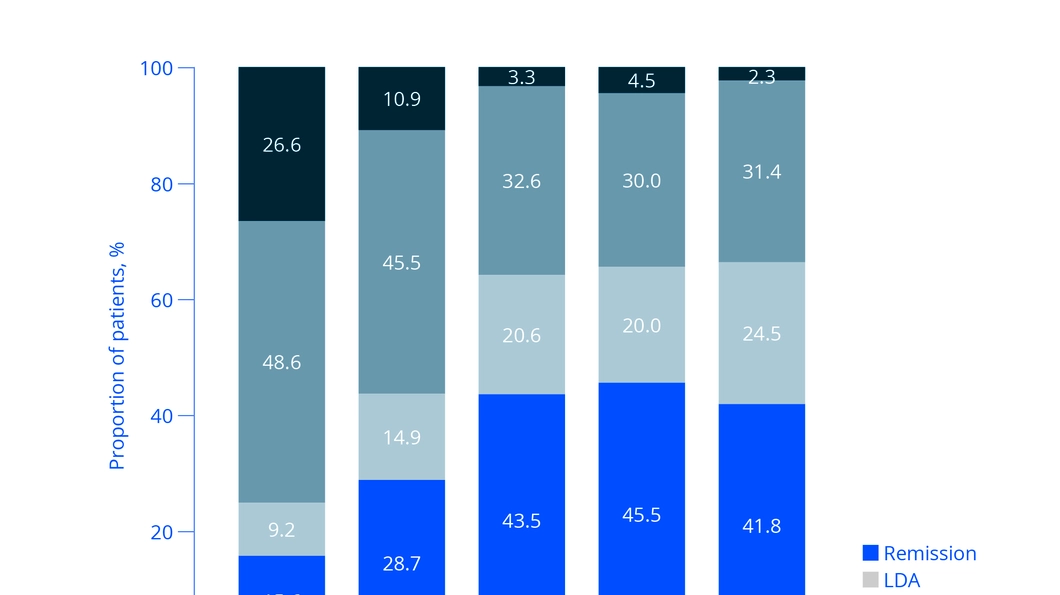
Subcutaneous methotrexate in Japanese patients with RA
Prof. Yoshiya Tanaka presented his study on the therapy of Japanese patients with RA with SC MTX 8. This study was funded by medac and laid the foundation for the approval of SC MTX in Japan. The first part of the study showed a trend of improved tolerability of SC MTX compared to oral MTX administration: Compared to oral MTX, less than half of the patients receiving SC MTX suffered from gastrointestinal side effects. In particular, fewer patients suffered from nausea when receiving SC MTX.
In the second part of the study, all patients received SC MTX, and the dose was successively increased on a patient-by-patient basis. A few months after the patients received increased SC MTX doses, 2 out of 3 patients achieved DAS28 (ESR) remission or low disease activity, which lasted until the end of the study (see Fig. 1) Tanaka summarised that the study showed the very good effectiveness and tolerability of SC MTX for the therapy of RA in Japanese patients.”
Methotrexate remains a cornerstone in RA therapy
In the following presentation, Prof. Andrea Rubbert-Roth presented interesting publications where the “gold standard" SC MTX shines once again. A recent study from Artacho et al 9, demonstrate that the gut microbiome may affect MTX metabolism and clinical response to MTX. A higher bacterial diversity in MTX-non responders included bacteria that more rapidly metabolise MTX. This result might explain the superiority of subcutaneously applied MTX, stated Prof. Rubbert-Roth in her talk. A prospective, multicentric observational study with 722 RA patients 10 on MTX pointed out the advantages about optimising MTX before escalating to bDMARDs: Increasing the dosage and using the SC route for MTX showed similar results as adding biological agents.
How could we identify difficult-to-treat RA patients early?
Finally, Dr. Alessandro Giollo, presented his retrospective study on difficult-to-treat (DTT) RA. 11 His publication shows that the lack of early MTX therapy and continued treatment with glucocorticoids are predictive features of DTT-RA. He pointed out that DTT RA affected up to 20% of RA patients, and that treatment-related factors in the initial management of RA may modify the risk of DTT-RA. For example delaying the introduction of MTX and prolonging use of GC therapy predict DTT-RA. Early use of MTX within the first 3 months after diagnosis may prevent especially persistent inflammatory refractory RA.
The medac symposium was very well attended, the room was completely filled with 250 participants, many interested physicians had to stay outside. Due to the wide range of information, the attending physicians were able to deepen their knowledge of MTX and its benefits in SC administration in a focused manner.
About metoject®/metex® PEN
Since 2014 – first in Germany, then followed by further European countries – the worldwide first methotrexate-filled autoinjector metoject®/metex® PEN has made it easier for patients in Germany and Europe with RA to apply methotrexate subcutaneously. metoject®/metex® PEN are manufactured by medac in Germany.
Find out more at www.metoject.com
- Smolen JS, Landewé R, Bijlsma J, et al. Ann Rheum Dis 2017;76:960–977.6.
- Schiff MH, et al. Ann Rheum Dis 2014;73:1549–1551.
- Braun J et al. Arthritis Rheum. 2008; 58(1):73-81.
- Kromann CB et al. Dermatolog Treat, 2015; 26(2): 188-190.
- Braun J et al, Arthritis Rheum 2008; 58:73-81.
- Harris E, Ng B. Eur J Rheumatol 2018; 5:85–91.
- Pardey N et al. Z Rheumatol 2021
- Tanaka Y, Okuda K, Takeuchi Y et al. Mod Rheumatol. 2022 Sep 2:roac103.
- Artacho A et al, Arthritis Rheumatol 2021, 73 (6): 931-942
- Gaujoux-Viala C et al. Rheumatology 2022; 61: 270-280.
- Giollo A et al. Rheumatology 2022 Oct 3:keac563.